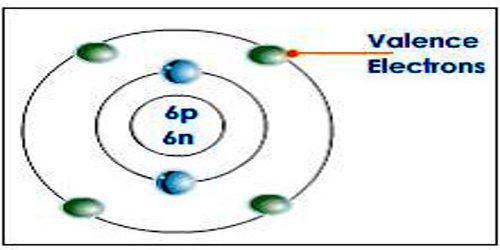
In chemistry and physics, a valence electron is an outer shell electron that is associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. Valence electrons are the total number of electrons present in the outermost shell of an atom (i.e. In outermost orbital). The valence electrons for a neutral atom is always definite, it cannot be varied (more or less) in any condition for a particular atom and may or not be equal to its valency.
Element Iodine - I
Valence Electrons The electrons in the last orbit which also determines mainly the electrical properties of the elements are known as valence electrons. It is well known to us that the outermost shell of an atom processes maximum 8 number of electrons. So the maximum number of valence electrons of an atom cannot be more than 8. Valence Electrons of all the elements in the Periodic Table Refer to graph, table and property element trend below for Valence Electrons of all the elements in the periodic table. We have shown the Valence Electrons of the elements for which reliable data is available. Valence Electrons Graph - Valence Electrons of all the elements in graph.
Comprehensive data on the chemical element Iodine is provided on this page; including scores of properties, element names in many languages, most known nuclides of Iodine. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Iodine Menu
- Iodine Page One
- Iodine Page Two
- Iodine Page Three
Overview of Iodine
- Atomic Number: 53
- Group: 17
- Period: 5
- Series: Halogens
Iodine's Name in Other Languages
- Latin: Iodum
- Czech: Jod
- Croatian: Jod
- French: Iode
- German: Jod - r
- Italian: Iodio
- Norwegian: Jod
- Portuguese: Iôdo
- Russian: Иод
- Spanish: Yodo
- Swedish: Jod
Atomic Structure of Iodine
- Atomic Radius: 1.32Å
- Atomic Volume: 25.74cm3/mol
- Covalent Radius: 1.33Å
- Cross Section (Thermal Neutron Capture) σa/barns: 6.2
- Crystal Structure: Orthorhombic
- Electron Configuration:
- 1s2 2s2p6 3s2p6d10 4s2p6d10 5s2p5
- Electrons per Energy Level: 2,8,18,18,7
- Shell Model
- Shell Model
- Ionic Radius: 2.2Å
- Filling Orbital: 5p5
- Number of Electrons (with no charge): 53
- Number of Neutrons (most common/stable nuclide): 74
- Number of Protons: 53
- Oxidation States:±1,5,7
- Valence Electrons: 5s2p5
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Iodine
- Electrochemical Equivalent: 4.7348g/amp-hr
- Electron Work Function:
- Electronegativity: 2.66 (Pauling); 2.21 (Allrod Rochow)
- Heat of Fusion: 7.824kJ/mol
- Incompatibilities:
- Ammonia, acetylene, acetaldehyde, powdered aluminum, active metals, liquid chlorine
- Ionization Potential
- First: 10.451
- Second: 19.131
- Third: 33
- Valence Electron Potential (-eV): -6.55
Physical Properties of Iodine
- Atomic Mass Average: 126.9045
- Boiling Point: 458.55K 185.4°C 365.7°F
- Coefficient of lineal thermal expansion/K-1: N/A
- Conductivity
- Electrical: 8.0E-16 106/cm Ω
Thermal: 0.00449 W/cmk
- Electrical: 8.0E-16 106/cm Ω
- Density: 4.93g/cc @ 300K
- Description:
- Halogen solid form: shiny, non-metallic, grayish-black flakes; gas: violet.
- Elastic Modulus:
- Bulk: 7.7/GPa
- Enthalpy of Atomization: 106.7 kJ/mole @ 25°C
- Enthalpy of Fusion: 7.76 kJ/mole
- Enthalpy of Vaporization: 20.88 kJ/mole
- Flammablity Class: Non-combustible solid
- Freezing Point:see melting point
- Heat of Vaporization: 20.752kJ/mol
- Melting Point: 386.65K 113.5°C 236.3°F
- Molar Volume: 25.74 cm3/mole
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 0.214J/gK
Regulatory / Health
- CAS Number
- 7553-56-2
- RTECS: NN1575000
- OSHAPermissible Exposure Limit (PEL)
- 1 ppm = 10.38mg/m3 @ 25°C & 1 atm
- Ceiling: 0.1 ppm
- OSHA PEL Vacated 1989
- Ceiling: 0.1 ppm
- NIOSHRecommended Exposure Limit (REL)
- Ceiling: 0.1 ppm
- IDLH: 2 ppm
- Routes of Exposure: Inhalation; Ingestion; Skin and/or eye contact
- Target Organs: Eyes, skin, respiratory system, central nervous system, cardiovascular system
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 0.057
- Bone/p.p.m: 0.27
- Liver/p.p.m: 0.7
- Muscle/p.p.m: 0.05-0.5
- Daily Dietary Intake: 0.1-0.2 mg
- Total Mass In Avg. 70kg human: 12-20 mg
Who / Where / When / How
- Discoverer: Bernard Courtois
- Discovery Location: Dijon France
- Discovery Year: 1811
- Name Origin:
- Greek: iôdes (violet).
- Abundance of Iodine:
- Earth's Crust/p.p.m.: 0.14
- Seawater/p.p.m.:
- Atlantic Suface: 0.0489
- Atlantic Deep: 0.056
- Pacific Surface: 0.043
- Pacific Deep: 0.058
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): N/A
- Sources of Iodine:
- Occurs on land and in the sea in sodium and potassium compounds. World production of iodine is around 12,000 tons. Primary producing areas are Chile and Japan.
- Uses of Iodine:
- Required in small amounts by humans. Once used as an antiseptic, but no longer due to its poisonous nature. Used as a disinfectant, in pharmaceuticals, dyes, catalysts and photography.
- Additional Notes:
Iodine Menu
- Iodine Page One
- Iodine Page Two
- Iodine Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Iodine - I. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/I.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/I.html'>echo Periodic Table of Elements: Iodine - I (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Iodine - I is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.
Learning Objectives
- Define valence electron.
- Be able to indicate valence electrons when given the electron configuration for an atom.
What makes a particular element very reactive and another element non-reactive?
A chemical reaction involves either electron removal, electron addition, or electron sharing. The path a specific element will take depends on where the electrons are in the atom and how many there are.
| Element Name | Symbol | Atomic Number | Electron Configuration |
| Lithium | Li | 3 | 1s22s1 |
| Beryllium | Be | 4 | 1s22s2 |
| Boron | B | 5 | 1s22s22p1 |
| Carbon | C | 6 | 1s22s22p2 |
| Nitrogen | N | 7 | 1s22s22p3 |
| Oxygen | O | 8 | 1s22s22p4 |
| Fluorine | F | 9 | 1s22s22p5 |
| Neon | Ne | 10 | 1s22s22p6 |
In the study of chemical reactivity, we will find that the electrons in the outermost principal energy level are very important and so they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements listed above, the two electrons in the 1 s sublevel are called inner-shell electrons and are not involved directly in the element’s reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the 2 s and the 2 p sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in s2p6, has eight valence electrons.
Summary
- Valence electrons are the outer-shell electrons of an atom.
- Valence electrons determine the reactivity of an atom.
Practice
Use the link below to answer questions about valence electrons:
Review
- Define valence electron.
- Define inner shell electron.
- How many valence electrons are there in fluorine?
- What are the 2s electrons in nitrogen?
- How many inner shell electrons are there in beryllium?
Glossary
I Valence Electrons
- inner-shell electrons: Those electrons that are not in the outer shell and are not involved in the reactivity of the element.
- valence electrons: The electrons in the highest occupied principal energy level of an atom.
I Core And Valence Electrons
Show ReferencesReferences
- User:Chemicalinterest/Wikipedia. http://commons.wikimedia.org/wiki/File:Cobalt_carbonate.JPG.
