All atoms of an element are identical. The atoms of different elements vary in size and mass. Compounds are produced through different whole-number combinations of atoms. A chemical reaction results in the rearrangement of atoms in the reactant and product compounds. There are three parts to an atom. Protons have a positive electrical charge and are found together. All atoms have a dense central core called the atomic nucleus. Forming the nucleus are two kinds of particles: protons, which have a positive electrical charge, and neutrons, which have no charge. All atoms have at least one proton in their core, and the number of protons determines which kind of element an atom is. For example, an oxygen atom. A chemist’s bestiary: An atom is a single nucleus with its electrons. An element is any of a number of atoms whose nuclei have the same number of protons. An isotope is an element with a defined number of neutrons, as well. A) positively charged, with the number of protons exceeding the number of electrons B) negatively charged, with the number of electrons exceeding the number of protons C) neutral, with the number of protons equaling the number of electrons D) neutral, with the number of protons equaling the number of electrons, which is equal to the number of neutrons E) neutral, with the number of protons.
- All Atoms Are In Constant Motion
- Everything To Know About Atoms
- All Atoms Are Exactly The Same
- All Atoms Are What
- Atomic model
- Basic properties
- The electron
- The nucleus
- Development of atomic theory
- The beginnings of modern atomic theory
- Studies of the properties of atoms
- Models of atomic structure
- Advances in nuclear and subatomic physics
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!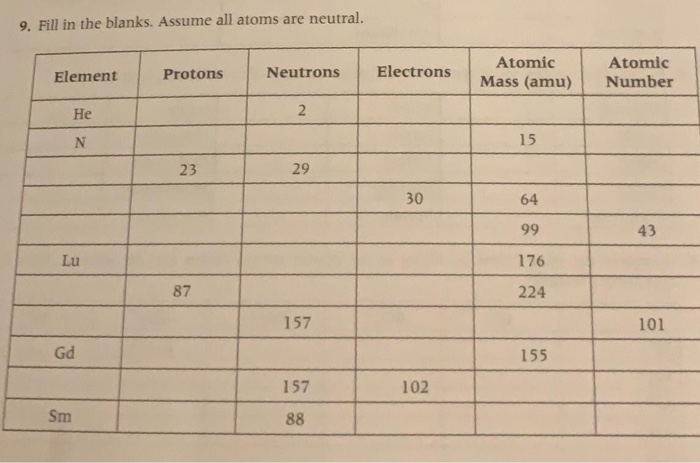
Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
Most of the atom is empty space. The rest consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. Electrons are attracted to any positive charge by their electric force; in an atom, electric forces bind the electrons to the nucleus.
Because of the nature of quantum mechanics, no single image has been entirely satisfactory at visualizing the atom’s various characteristics, which thus forces physicists to use complementary pictures of the atom to explain different properties. In some respects, the electrons in an atom behave like particles orbiting the nucleus. In others, the electrons behave like waves frozen in position around the nucleus. Such wave patterns, called orbitals, describe the distribution of individual electrons. The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells.
This article opens with a broad overview of the fundamental properties of the atom and its constituent particles and forces. Following this overview is a historical survey of the most influential concepts about the atom that have been formulated through the centuries. For additional information pertaining to nuclear structure and elementary particles, seesubatomic particles.
Atomic model
Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Attempts to separate these smaller constituent particles require ever-increasing amounts of energy and result in the creation of new subatomic particles, many of which are charged.
As noted in the introduction to this article, an atom consists largely of empty space. The nucleus is the positively charged centre of an atom and contains most of its mass. It is composed of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms. Other subatomic particles may be found in association with these three types of particles. They can be created only with the addition of enormous amounts of energy, however, and are very short-lived.
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10−10 metre. The radius of an atom measures 1–2 Å. Compared with the overall size of the atom, the nucleus is even more minute. It is in the same proportion to the atom as a marble is to a football field. In volume the nucleus takes up only 10−14 metres of the space in the atom—i.e., 1 part in 100,000. A convenient unit of length for measuring nuclear sizes is the femtometre (fm), which equals 10−15 metre. The diameter of a nucleus depends on the number of particles it contains and ranges from about 4 fm for a light nucleus such as carbon to 15 fm for a heavy nucleus such as lead. In spite of the small size of the nucleus, virtually all the mass of the atom is concentrated there. The protons are massive, positively charged particles, whereas the neutrons have no charge and are slightly more massive than the protons. The fact that nuclei can have anywhere from 1 to nearly 300 protons and neutrons accounts for their wide variation in mass. The lightest nucleus, that of hydrogen, is 1,836 times more massive than an electron, while heavy nuclei are nearly 500,000 times more massive.
All Atoms Are In Constant Motion
Basic properties
Atomic number
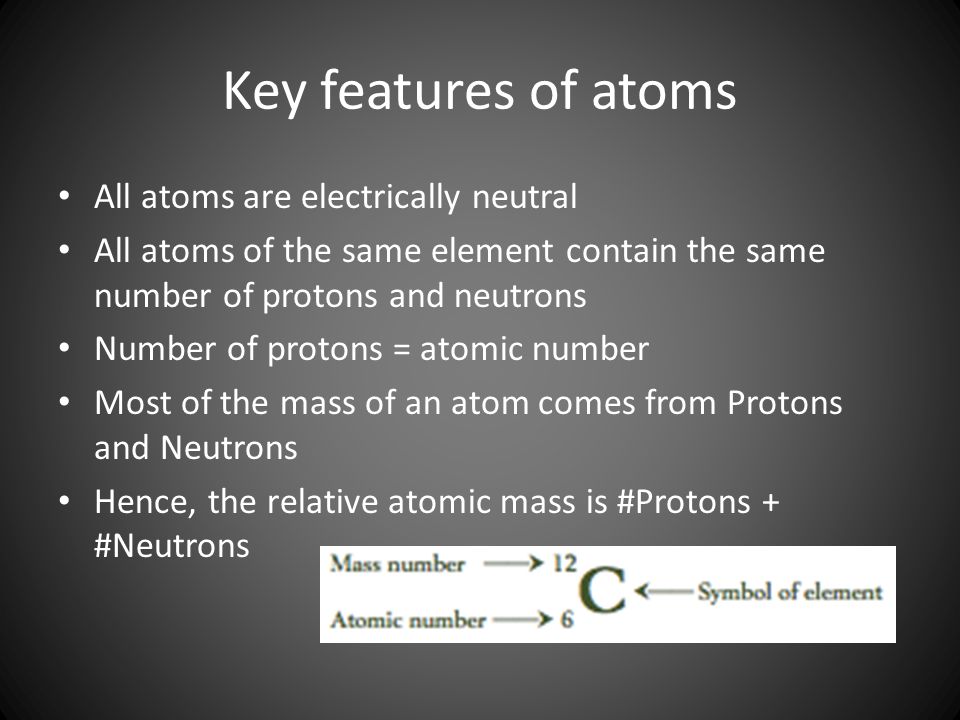
The single most important characteristic of an atom is its atomic number (usually denoted by the letter Z), which is defined as the number of units of positive charge (protons) in the nucleus. For example, if an atom has a Z of 6, it is carbon, while a Z of 92 corresponds to uranium. A neutral atom has an equal number of protons and electrons so that the positive and negative charges exactly balance. Since it is the electrons that determine how one atom interacts with another, in the end it is the number of protons in the nucleus that determines the chemical properties of an atom.

- key people
- related topics
Although life on Earth may seem rather stable and unchanging—the tide goes in and out, the Sun rises and sets, and the months bleed on the same as always—in the grand scheme of things, our universe is actually a rather dynamic place.
Everyday, millions of stars are born and die, and in the end, the same thing will happen to our own Sun.
A few billion years from now, as our star begins to transition into a red giant, temperatures on our world will increase, and life will be extinguished. Just a few billion years after that, once the Sun exhausts its supply of material to sustain nuclear fusion, it will begin its death throes. When this happens, it will cast off its outer layers and, eventually, fade into darkness.
Nothing in our universe is eternal…or is it?
Atoms are the building blocks of matter. They, quite literally, make our universe what it is. When we die, our bodies do not turn into nothing; rather, they are broken down into their constituent parts and recycled into the ecosystem. In short, our atoms go on long after we are gone.
But just how long can atoms last? Will they eventually be broken down into…nothing?
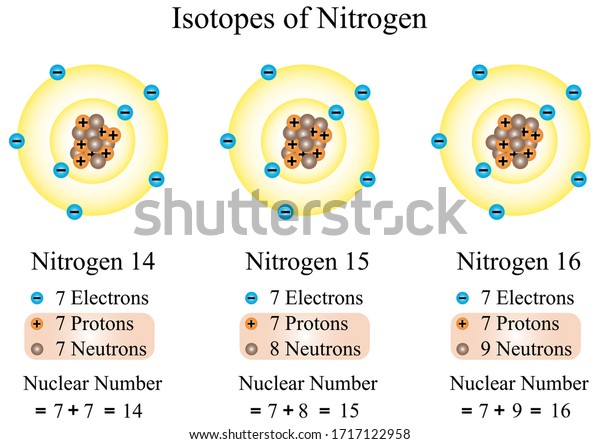
To answer this question, you need to understand a little bit about how atoms work. As you may know, atoms contain protons and neutrons, and they are surrounded by a “shell” of electrons. The number of electrons that are found in the cloud are equal to the number of protons. This helps create stability.
Everything To Know About Atoms
Ultimately, the number of protons is what determines the atomic number. For example, helium has two protons, so its atomic number is two (and it appears second on the periodic table of elements). The number of neutrons that are found in an atom are generally consistent, but not always. And if an atom doesn’t have the “correct” number of neutrons, sometimes, the atom may lose a neutron (kind of like how you lose a sock in the wash). When this happen, the atom becomes unstable and, in an attempt to become a stable atom, it shoots off subatomic particles. Most often, the atom will shoot off an electron.
This is how atoms breakdown.
Anytime that you have a heavy atom, there is some risk that it will spontaneously start to break down into smaller particles. This is known as “radioactive decay.” This is just a very basic breakdown. Please see the link for more on radioactive decay.
To return to the point at hand, unfortunately, this is a stochastic process (which means that it has “a random probability distribution, or pattern that may be analyzed statistically, but may not be predicted precisely”). In other words, we can’t pinpoint exactly when a breakdown will occur – when a subatomic particle will be shot off; however, since we can analyze the pattern, we can determine how many atoms will decay over an average time, which is called the”half-life,” and it is a very reliable estimate.
All Atoms Are Exactly The Same
Since an atom has a finite number of protons and neutrons, it will generally emit particles until it gets to a point where its half-life is so long, it is effectively stable. For example, Bismuth-209 is believed to have the longest decay rate. It undergoes something known as “alpha decay,” and it’s half-life is over a billion times longer than the current estimated age of the universe.
So for all intents and purposes, Bismuth-209 is basically eternal.
That said, true eternal life depends on whether or not protons can decay. Some scientists have put forth hypotheses related to this, and it is referred to as “proton decay” (a hypothetical form of radioactive decay). According to one idea, the Georgi–Glashow model, protons transition into a positron and a neutral pion, which then decays into 2 gamma ray photons. Estimates put the half-life for protons at 1.29×1034 years.
That, if you don’t know, is a super long time; however, there is no experimental evidence to confirm proton decay. But research that is being conducted at some of the world’s mega laboratories may, with a bit of luck and hard science, reveal something in the future.
As a Futurism reader, we invite you join the Singularity Global Community, our parent company’s forum to discuss futuristic science & technology with like-minded people from all over the world. It’s free to join, sign up now!
All Atoms Are What
Share This Article
